往期刊物2025
卷册: 15, 期号: 17
生物化学
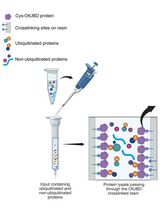
Use of a High-Affinity Ubiquitin-Binding Domain to Detect and Purify Ubiquitinated Substrates and Their Interacting Proteins
利用高亲和力泛素结合结构域检测和纯化泛素化底物及其互作蛋白
生物信息学与计算生物学
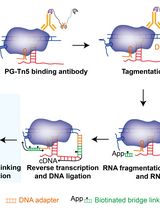
Simultaneous Capture of Chromatin-Associated RNA and Global RNA–RNA Interactions With Reduced Input Requirements
低样本量条件下同时捕获染色质相关RNA及全局RNA–RNA互作
生物物理学
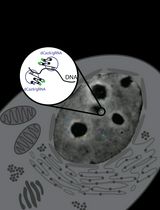
Real-Time Imaging of Specific Genomic Loci With CRISPR/dCas9 in Human Cells Using CRISPRainbow
利用CRISPRainbow在人体细胞中实时成像特定位点基因组
癌症生物学
NanoPDLIM2-Based Combination Therapy for Lung Cancer Treatment in Mouse Preclinical Studies
基于NanoPDLIM2的小鼠肺癌前临床联合治疗研究
免疫学
Quantitative Microscopy for Cell–Surface and Cell–Cell Interactions in Immunology
免疫学中用于研究细胞表面与细胞间相互作用的定量显微技术
Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
神经科学
Ultrafast Isolation of Synaptic Terminals From Rat Brain for Cryo-Electron Tomography Analysis
用于冷冻电子断层成像分析的大鼠脑突触末端超快速分离
Training Mice to Perform Attentional Set-Shifting Under Head Restraint
限制头部条件下小鼠注意设置转换训练
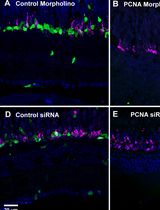
Efficient Gene Knockdown in Adult Zebrafish Retina by Intravitreal Injection
玻璃体腔注射实现成年斑马鱼视网膜的高效基因敲低
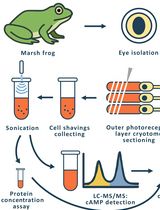
Time-Resolved cAMP Level Determination in Frog Retina Samples Using LC–MS/MS
基于LC–MS/MS的蛙视网膜样品cAMP水平的时间分辨检测
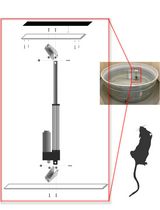
Constructing and Implementing a Low-Cost On-Demand Morris Water Maze Platform
低成本按需构建与应用的Morris水迷宫平台
植物科学
PhosphoLIMBO: An Easy and Efficient Protocol to Separate and Analyze Phospholipids by HPTLC From Plant Material
PhosphoLIMBO:一种基于HPTLC的植物材料磷脂分离与分析简便高效方法
New Approach to Detect and Isolate Rhamnogalacturonan-II in Arabidopsis thaliana Seed Mucilage
检测和分离拟南芥种子黏液中鼠李半乳糖醛酸Ⅱ的新方法
干细胞
Simultaneous RNA Fluorescent In Situ Hybridization and Immunofluorescent Staining of Mouse Muscle Stem Cells on Fresh Frozen Skeletal Muscle Sections
新鲜冷冻骨骼肌切片上小鼠肌肉干细胞的RNA荧光原位杂交与免疫荧光染色同步检测